
Crystals to cosmetics, this post is about various minerals which have been used, without chemical alteration, as makeup.
OCHRE
Evidence of the use of minerals to adorn human skin has been gathered back to around 7000 years ago, although there is little doubt that the use of ochre could reach back to early paleolithic times.

The use of ochre by folk in native communities ranges from the subtle, seen at left "Boy Chief - Ojibbeway" (Calin 1843), to at right, "Namibian Himba lady" (Yves Picq 2007), with the "slap it on" approach usually observed at Newcastle quayside.
Ochre is a family of earth pigments, which includes yellow ochre, red ochre, purple & blue ochre, sienna, and umber. The major ingredient of all the ochres is limonite, iron(III) oxide-hydroxide, which gives them a base yellow colour.
 Limonite, Trinidad Mine, Benalmádena, Málaga, Andalusia, Spain
Limonite, Trinidad Mine, Benalmádena, Málaga, Andalusia, Spain
Yellow ochre, FeO(OH)·nH2O, is a hydrated iron hydroxide (limonite) also called gold ochre.
Red ochre, takes its reddish colour from the mineral hematite, Fe2O3, which is an anhydrous iron oxide.
 Hematite Var Kidney Ore, West Cumberland Iron Field, Cumbria, United Kingdom.
Hematite Var Kidney Ore, West Cumberland Iron Field, Cumbria, United Kingdom.
Purple ochre, is identical to red ochre chemically but of a different hue caused by different light diffraction properties associated with a greater average particle size.
Brown ochre, goethite, α-Fe3+O(OH), is a partly hydrated iron oxide.
 Goethite, Filón Sur, Tharsis, Huelva, Andalusia, Spain
Goethite, Filón Sur, Tharsis, Huelva, Andalusia, Spain
Other iron minerals occasionally present in ochre include Lepidocrocite γ-Fe3+O(OH) Maghemite Fe3+2O3 Magnetite Fe2+Fe3+2O4 and Siderite FeCO3
Sienna contains both limonite and a small amount of manganese oxides (less than five percent), which makes it darker than ochre.
Umber pigments contain a larger proportion of manganese oxides (five to twenty percent) which make them a dark brown.
The black manganese oxides are found as psilomelane, pyrolusite, and the generic "wad."
 Manganese oxide dendrites on a limestone bedding plane from Solnhofen, Germany.
Manganese oxide dendrites on a limestone bedding plane from Solnhofen, Germany.
Blue Ochre, contains the mineral Vivianite

Vivianite, Tomokoni mine, Machacamarca District, Potosi Department, Bolivia
The crystal of vivianite above is the beautiful green of freshly mined examples. Exposure to sunlight and air causes an oxidation process which dramatically causes a colour change towards a dark blue. Eventually the vivianite Fe2+3(PO4)2·8H2O changes to a new species, metavivianite Fe2+Fe3+2(PO4)2(OH)2·6H2O
Of course crystals like the above were not powdered down to make blue ochre. Mud rich in phosphates occurs naturally, and can be quite a striking blue. A photo from the Barnes excavations of Stainton Beck, just under the Auroch skulls, shows "blue earth" here. It's due to vivianite forming from organic remains.
Going way back to ancient Egyptians, the likes of Cleopatra demanded classier cosmetics than smears of iron oxide and phosphate mud. Her eye-shadow makeup was made from a mineral worth more than its weight in gold, Lazurite.

ULTRAMARINE
Ultramarine is a deep blue pigment which was originally made by grinding lazurite bearing Lapis lazuli into a powder.
 Lazurite crystal in marble matrix, Sar-e-sang, Badakhshan Province, Afghanistan
Lazurite crystal in marble matrix, Sar-e-sang, Badakhshan Province, Afghanistan
The blue color of the pigment is due to the absorption spectrum of trisulfur radical anions S3- which have an unpaired electron, and are trapped within sodalite cages -
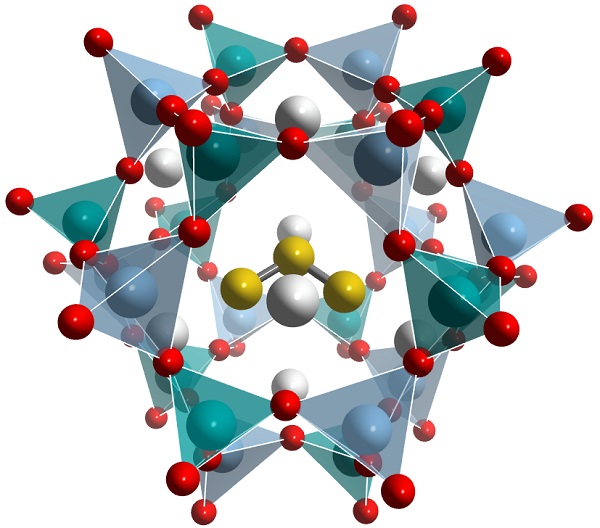 Lazurite structure, rendered using Vesta software, by chemicalstructure.net The highly reactive chromophore ion, also known as thiozonide, is the trio of yellow balls.
Lazurite structure, rendered using Vesta software, by chemicalstructure.net The highly reactive chromophore ion, also known as thiozonide, is the trio of yellow balls.
Besides when confined in the sodalite cage of lazurite, S3- anions are only "stable" in aqueous solution under a pressure of 0.5 GPa (73,000 psi), and are expected to occur naturally at depth in the earth's crust where subduction or high pressure metamorphism occurs. Thiozonide is probably important in movement of copper and gold in high pressure hydrothermal fluids.
Cleopatra's eye-shadow tastes occasionally drifted to the verdant green of Malachite Cu2CO3(OH)2
 Malachite, Mashamba West Mine, Katanga, Democratic Republic of the Congo
Malachite, Mashamba West Mine, Katanga, Democratic Republic of the Congo
Cleopatra's eye-liner was thick, black and poisonous. The same formulation is now in use by millions in North Africa, the Middle East, Anatolia and South Asia...
KOHL
Kohl is ground up Galena PbS and/or Stibnite Sb2S3. Lead and antimony compounds are a tad hazardous to use around moist areas of the body, such as tear-ducts or the conjunctiva. Modern heavy-metal poisoning is a real problem in the Middle-East, because of the use of traditionally made kohl eye-liner. Users quite rightly point out that kohl prevents eye infections. Lead kills bacteria faster than it destroys folk's health.
 Galena crystal, Sweetwater Mine, Missouri. Also Theda Bara in the film Cleopatra (1917) and for e-Rocks' Mark, an Egyptian alabaster kohl pot, which possibly dates to Dynasty XII in the Middle Kingdom.
Galena crystal, Sweetwater Mine, Missouri. Also Theda Bara in the film Cleopatra (1917) and for e-Rocks' Mark, an Egyptian alabaster kohl pot, which possibly dates to Dynasty XII in the Middle Kingdom.
 Stibnite crystal clusters, Baiut Mine, Cavnic, Romania. Plus a double cosmetic tube (dilekythos) used for kohl, 4th Century AD
Stibnite crystal clusters, Baiut Mine, Cavnic, Romania. Plus a double cosmetic tube (dilekythos) used for kohl, 4th Century AD
VERMILION
This brilliant red or scarlet pigment was made from the powdered mineral cinnabar, HgS
 Cinnabar, Tongren Mine, Tongren, Guizhou Province, China
Cinnabar, Tongren Mine, Tongren, Guizhou Province, China
Comanche Native Americans discovered the rich cinnabar deposits at the Terlingua quicksilver district of southwestern Texas. As blood-red war-paint, this stuff was the business, guaranteed to put the willies up any palefaces and well worth the mercury poisoning...
GLIMMER & SHIMMER
Widely used in cosmetics to give a shimmering sparkly effect, finely flaked mica KAl2(AlSi3O10)(OH)2 works well as a natural glitter.
 Mica books on quartz, Skardu, Gilgit-Baltistan, Pakistan
Mica books on quartz, Skardu, Gilgit-Baltistan, Pakistan
PEARLESCENCE
Bismoclite BiOCl is a rare mineral, and it is doubtful whether any cosmetics have ever been produced from it. However, it is naturally occurring bismuth oxychloride, a chemical used in cosmetics since antiquity, notably in ancient Egypt.
 Crystals of orange Bismoclite, La Fossa Crater, Vulcano Island, Aeolian Islands, Sicily, Italy
Crystals of orange Bismoclite, La Fossa Crater, Vulcano Island, Aeolian Islands, Sicily, Italy
Light wave interference from its plate-like structure gives a pearly iridescent light reflectivity similar to nacre. Still used today, "a pearly pigment found in eye shadow, hair sprays, powders, nail polishes, and other cosmetic products"
 BiOCl structure by Materialscientist
BiOCl structure by Materialscientist
The structure of bismuth oxychloride can be thought of as consisting of layers of Cl−, Bi3+ and O2− ions (in the image Bi = grey, O = red, Cl = green). These ions are ordered as Cl-Bi-O-Bi-Cl-Cl-Bi-O-Bi-Cl, i.e., with alternating anions (Cl−, O2−) and cations (Bi3+). The layered structure gives rise to the pearlescent properties of this material.
FOUNDATION
Modern foundation cosmetics and face-powders come in a variety of colours and shades. In the past however, there was only one fashionable complexion, deathly white. During the Renaissance up until the 20th century, European social climbers found it necessary to apply makeup to look as pale as the aristocracy, who did no labor in the sun. To this end, various white pigments were applied -


Above from Instructions and Cautions Respecting the Selection and Use of Perfumes, Cooley, 1873
A nice mineral has historically been used on the skin, which isn't dangerous, and is cosmetically used today, Talc Mg3Si4O10(OH)2
 Crystallised talc from the Zederhaus Valley, Lungau, Salzburg, Austria
Crystallised talc from the Zederhaus Valley, Lungau, Salzburg, Austria
And lastly, a mineral which has no toxicity problems at all -
GOLD
 Native Gold, Hopes Nose, Torquay, Devon, United Kingdom
Native Gold, Hopes Nose, Torquay, Devon, United Kingdom

Above, 'The Golden Man', 1599 engraving by Theodor de Bry, and the Muisca raft (600 - 1600 AD), a one-pour lost-wax casting in gold. They represent the ceremony of investiture of the Muisca chief, which used to take place at Lake Guatavita, near present-day Bogotá. During this ritual, the heir to the chieftainship (zipa) covered his body with gold dust and jumped into the lake along with gold offerings and emeralds to the gods.
Although gold is non-toxic, an urban myth about it's lethality as a makeup germinated after the release of the film Goldfinger in 1964. Shirley Eaton, the actress who played Bond girl Jill Masterson, was in no danger of "skin suffocation," although her knickers look ruined....

References -
The S3– Ion Is Stable in Geological Fluids at Elevated Temperatures and Pressures, Pokrovski & Dubrovinsky, 2011
Stainton Beck Blue Earth, Barnes, 2011
Instructions and Cautions Respecting the Selection and Use of Perfumes, Cooley, 1873
And Now For Something Completely Different