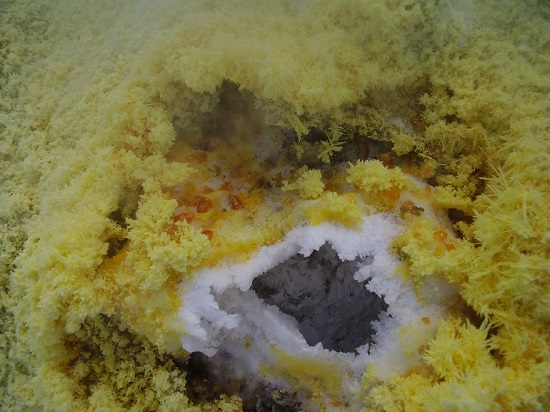
The La Fossa crater on the Aeolean Island of Vulcano has spectacular fumaroles. This one is venting gas at around 400°C, surrounded by an aureole of sublimates; white salammoniac and 4 allotropes (or polymorphs) of sulphur -
α-sulphur, or orthorhombic sulphur - the needles and solidified, previously molten sulphur cooled to < 95.3 °C
β-sulphur, or monoclinic sulphur - the solidified, previously molten sulphur at > 95.3 °C
λ-sulphur, the light yellow mobile liquid drops of sulphur, just melted at 119.6 °C
π-sulphur, the darker coloured, more viscous liquid at > 159°C

During dry periods, the (very soluble) salammoniac sublimates can develop into dendritic crystals >2cm surrounding the fumaroles. They are extremely delicate and therefore need complete freedom from wind to develop into this beauty.

The salammoniac (NH4Cl) is crystallising not by sublimation, but it is actually being formed at the neck of the fumarole, from cooling hydrogen chloride and ammonia gasses reacting. The dendrites grow along the octahedral axis (3 fold axis) 111, perpendicular to the octahedral faces, with fans of spikes at 0, 120 & 240 degrees.
I took the photos above whilst camping on the volcanic island of Vulcano in 2007. They’re of beautifully crystallised salammoniac (ammonium chloride). The crystals form around the vents of active fumaroles in the La Fossa crater. Being very soluble in water, their growth to this degree is only possible during an extended dry spell.
The next year I returned to this spectacular Aeolian Island, again to camp out next to the shore of the Tyrrhenian Sea. The fumaroles in '08 showed only minimal crystallisation (although stunning though a loupe), due to frequent thunderstorms. As a side note, one morning after a particularly violent (and quite frightening inside our tent) thunderstorm, I climbed the volcano to examine the summit's copper lightning conductor. Electrical fusing was obvious, an anthropomorphic fulgurite, below.

In '07 I had carefully collected a selection of the most robust examples of sal ammoniac crystals. Unfortunately a combination of their extreme fragility and deliquescence caused these beautiful crystals to turn into the disaster below (storage in my garden shed for 11 years was unwise).

Should I ever get round to selling my mineral collection, these specimens are of little worth. I decided to eat the specimens. The mineral salammoniac is ammonium chloride, an interesting chemical. When heated to 315 °C, it dissociates into ammonia and hydrogen chloride gases. It looks like it sublimes, but it's a decomposition, the gases recombining upon cooling, forming ammonium chloride again. I decided to use this "sublimation" to purify my volcanic samples, since I wanted to taste the stuff without any possibility of thallium poisoning. Enter the Kraken -

I'd been researching the giant squid (Architeuthis) and discovered that calamari made from these big bastards tastes bloody awful. Unless you're Finnish. Apparently giant squid have a solution of ammonium chloride permeating their bodies. The liquid is much less dense than sea-water and enables them to achieve neutral buoyancy. The Finland connection? A favourite sweety of the Scandinavians is salmiakki - salty liquorice. This stuff isn't salty as such, more astringent due to being liberally concocted with ammonium chloride. Another gem of knowledge I had been blissfully unaware of. I had to try some. The liquorice, not the calamari architeuthis.
Rather than buying some salmiakki from eBay, I thought it would be better to make my own, using the product of my favourite volcano. One of the problems with mineral collecting from Vulcano's La Fossa crater is the possibility of thallium poisoning. Vulcano is one of the best places in the world to collect minerals (103 at the last count), it's the type locality (the place where a mineral is first found) for some 33 exotic minerals. The thallium mineral lafossaite is found there. It's a mineral composed of thallium, chlorine and bromine, and is highly poisonous
 Left, Lafossaite. Right, a bottle of pure thallium chloride
Left, Lafossaite. Right, a bottle of pure thallium chloride
It's formed at the neck of similar, but higher temperature, fumaroles to where my sal ammoniac was collected. So my source of liquorice flavouring was rather suspect, health-wise.
Thallium poisoning is decidedly nasty. It's a highly debilitating poison should you survive it - hair loss and the sensation of walking on hot coals being the least of your problems. Besides being used by innumerable amateur murderers as "Inheritance Powder," it was the preferred poison used by Saddam Hussein to punish "dissidents." The British South African Police used it for some unfathomable reason against Zimbabwian freedom fighters. Incredibly the French Secret Service assassinated the president of Cameroon using it. I didn't want to use it on myself, so I decided to purify my sal ammoniac, just in case…
Firstly, I dissolved the mineral in water, so that any tiny crystals of undesirable nature could be filtered out. I then boiled the solution to lose water, and form a more concentrated solution. Ammonium chloride is highly soluble, and also has a high degree of difference between solubility at high and low temperatures. This enables a rather beautiful experiment to be performed with the filtrate (the Test Tube Snowstorm). I didn't video my efforts, but this by wymanthescienceman shows the effect admirably -
Note that instead of three dendrite fans at 120 degrees emergent from the primary needles, like the La Fossa crystals, the snowstorm crystals have fans on the cube axis (4 fans 90 degrees apart on 001, perpendicular to the cube face). This crystal form is apparent in many of the specimens illustrated in the next pic, below. Hopefully I've got the 111 vs. 001 crystal explanations right; I'm no crystallographer, and am open to correction in the comments below :)
Next, the "sublimation" or recrystallisation by dissociation. Hopefully this would leave behind any remaining lafossaite (fine particulates which may have passed through my Non-Analytical Whatman filter paper…. some bog-roll.) The boiling point of pure thallous chloride is 720 °C, some three hundred degrees above ammonium chloride's dissociation temperature. However, I was aware of a rather worrying paper on the transport of lafossaite at considerably lower temperatures…..
When I say worrying, I wasn’t really…. You’d have to eat quite a few grams of lafossaite before being poisoned. It’s very poorly soluble compared with the “inheritance powder,” thallium sulphate. I could have eaten the raw salammoniac specimens with no danger, but then wouldn’t have seen the snowstorm, or the weird sight of the dissociation.
I heated the salammoniac in a tuna tin with a butane torch. Above it I held a billycan full of ice. The white mineral just…. disappeared! No melting, it just goes, and reappears as an eerie white smoke a couple of centimeters above where it was. The billycan placed close to the dissociating mineral soon got coated with the “sublimate.” None of the beauty of the Vulcano dendrites, the rapidity of formation preventing the development of visible crystals.
And so, once the white powder was incorporated into some liquorice bought from our local corner shop, my volcanic salmiakki was ready for consumption. Horrible. I’m going to forgo the temptations of calamari architeuthis.
 Above, a selection of Salammoniac crystals, previously sold by e-Rocks, from various localities
Above, a selection of Salammoniac crystals, previously sold by e-Rocks, from various localities
e-Rocks has recently blogged about salammoniac, concerning a deposit in Chile which had been mistaken for Kröhnkite. For any salammoniac connoisseurs who just can't get enough, here's where Finnish liquourice stuffers of old got their fix, before the Industrial Revolution -
First, the Egyptian method, using oxen dung and camel piss. Following that, mining native salammoniac from the Tianshan Volcanic Group, in Northwest China, by naked natives in winter.
This abriged excerpt from The Encyclopaedia Britannica, seventh edition, 1842
AMMONIAC, SAL, a saline substance formerly much used in dyeing and some other arts. At present not much of it is employed in this country, most of the sal-ammoniac manufactured in Great Britain being sent to Russia.
Sal-ammoniac is usually in the form of a hard white cake opaque or only slightly translucent. Its taste is cooling saline and rather disagreeable; though it has been occasionally employed as a seasoner of food. The specific gravity is 1.441 according to the mean of the experiments of Wallerius Watson and Kirwan. It requires more than three times its weight of cold water dissolve it. By proper evaporation the sal-ammoniac may be obtained in crystals.
The primary form is considered to be the cube; but it crystallizes also in octahedrons, and in a figure bounded by 24 trapeziums, by replacing the angles of the cube by three faces. These, when they increase very much, cause faces of the cube to disappear, and thus form a 24 sided figure known in mineralogy by the name of leucite crystal. Sal-ammoniac often appears in the state of four sided pyramids, or of thin plates, like sword blades. It often assumes the form of plumose crystals, consisting of long hexahedral pyramids attached to a stem.
When exposed to a moist atmosphere, it gradually absorbs water and deliquesces, though very slowly. When heated, it sublimes unaltered in a white smoke, having peculiar smell very characteristic of sal-ammoniac. If cold body be presented to this smoke, the sal-ammoniac condenses on it and forms a white crust. When thus sublimed, it has the property of carrying along with it various bodies, which when heated by themselves, are perfectly fixed. Thus, if it be mixed with gold leaf, filings of iron or oxide of copper, these substances rise with it, and tinge the sal-ammoniac purple, yellow or red.
Sal-ammoniac occurs native in Bucharia, and probably in other parts of the world. It is found also in small quantities near volcanoes, being formed during the extraordinary convulsions of these mountains. This fact seems in corroboration of an opinion advanced by some mineralogists, that the principal fuel of volcanoes is pit-coal, and that they are always moistened with sea water; for sal-ammoniac is sublimed in small quantities during the burning of London bricks, the sand of which is from the sea shore, while the fuel is pit-coal.
Egypt is the country where sal-ammoniac was first manufactured, and from which Europe for many years supplied with it. Almost the only fuel used in Egypt is the dung of cattle. This is collected during the first four months of the year, when the cattle feed on spring grass, which in Egypt is a kind of clover. It is dried, and sold to common people as fuel. The soot from this fuel is carefully collected and sold to the sal-ammoniac makers, who only work during the months of March and April; for other periods of the year the dung of their cattle is not fit for their purpose. An oblong oven is built of bricks and moist dung, as long again as broad, and of such a that the outside or flat part of the top of the arch may hold fifty glass vessels, ten in length and five in breadth, each vessel having a cavity left for it in the brick work the arch. These glass vessels are globular, with a neck an inch long and two inches wide. In general they are about eighteen inches in diameter. Each vessel is coated over with a fine clay found in the Nile, and afterwards with straw. They are filled two thirds with soot, and put into their holes at the top of the oven. At first a gentle fire is raised, and the temperature is gradually increased to the highest degree, at which it is kept for three days. A smoke with a sourish smell, not unpleasant, issues first from the glasses, then the salt sublimes, and coats the upper part of the vessel.
It was long supposed that camel's urine and camel's dung were essential for the success of the above process; but this is a mistake. The dung of black cattle, horses, sheep, goats &c are all used promiscuously. The dung of these animals contains the sal-ammoniac ready formed. This depends upon the food which the animals live on, and accordingly it is only fit for thepurpose at one season of the year. The soot contains the sal-ammoniac likewise ready formed, and merely mixed with a quantity of charcoal, oil, &c. from which it is freed by sublimation. Chaptal informs us that he found sal arnmoniac in the soot obtained by burning the dung of cattle that had fed on the saline plants in the marshes near the Mediterranean. Thus the Egyptian method of obtaining sal-ammoniac is the simplest possible.

The following excerpt is from "On The Chains of Mountains and Volcanos of Central Asia," by Baron A. Von Humboldt. Printed in The Asiatic Journal and Monthly Miscellany, Volume 4, 1831.



If you've made it this far, the ammonium chloride is getting to you! Just one more occurrence of salammoniac, this time from my neck of the woods -
From Readings in Natural Philosophy, 1828


And Now for Something Completely Different
A Textbook of Assaying: For the Use of Those Connected with Mines., by Cornelius Beringer and John Jacob Beringer, 1904
UK Mining Document Library Loads of great information from AditNow
______________________________