
Thomas Edison had a superb mineral collection. He bought it in 1890 for $8000 (in today’s real money £185k). It was the personal collection of the notable self-taught mineralogist, gemmologist & Vice President of Tiffany & Co, George Frederick Kunz (the pink gem variety of spodumene is named after him).

Kunz had his mineral collection professionally catalogued prior to the sale. This was done by renowned collector & dealer George English (discoverer of penfieldite Pb2Cl3(OH), the rare lead hydroxychloride) -

The stock of Geo. L. English & Co. was described, "a truly wonderful assortment of Arizona wulfenites, vanadinites, azurites, and malachites in addition to fine crystallized Franklin fowlerites.” His ad in the May 1889 issue of The Exchangers' Monthly stated: "We deal exclusively in minerals; no rocks, fossils, Indian relics or curiosities" and "It is universally acknowledged that we have the finest and most complete stock of minerals in the United States."
Mr. English himself wrote out the inventory for Mr. Kunz’s collection, his distinctive script can be seen in the excerpts below. Presumably his valuation at $8000 was a good figure, given that much later English wrote the classic paper, “The Scientific Valuation of Minerals.”



With the prodigious provenance provided by Kunz & English, it’s interesting to note that the group “tungstates” does not include scheelite. Although the online scanned inventory is not clearly legible, I can't find scheelite anywhere, a crying shame for a comprehensive collection of some 3500 mineral specimens. And possibly, had Edison ever examined a scheelite specimen, he could have realised that an answer to his light-bulb needs lay in his folly of a gold mine, more of which later.
Blowing 8 grand on Kunz’s collection was nothing, Edison wasted millions on various mineral follies. His laboratory observations of the properties of some high-value minerals led him to believe that his scientific expertise could be used for their extraction and concentration from low-yield ores. Unfortunately Edison had no scientific expertise. At all.
To illustrate his level of laboratory skill, just look at the following movie clip & photos of the Great Man. He is attempting to use one of the most basic items of chemistry lab equipment, the wash-bottle.


Thomas Edison grasps a wash-bottle. He does not appear to grasp its function. To operate (looking at the LHS picture), you blow down the right-hand side upper tube. The washing agent (distilled water, organic solvent, or dilute acid) squirts out of the left-hand side lower tube, which is tapered to a nozzle. What Edison is up to is difficult to fathom, other than presenting his fellow workers with a poisoning risk by slowly dribbling the wash out of the mouthpiece.
Given this level of understanding of a basic tool’s operation, it’s obvious why Edison surrounded himself with workers having brains, experience and skills. They laboured hard to perfect his “inventions.” Under the leadership of Edison they successfully copied and improved other folks’ ideas & designs, such as Joseph Swan’s light bulb. This was accomplished even with Edison’s “veritable contempt for book learning and mathematical knowledge” (quote from Nikola Tesla). But when the great man himself had an original thought, particularly concerning something he knew little about, like minerals & mining,.... Well,... you can’t polish a turd.
In no particular order, here’s a few of Edison’s mineralogical adventures, starting with the noble metal -
Extraction of Native Gold by Electrostatic Levitation
Edison became aware that several gold mines had considerable reserves, but lacked the nearby water for washing and sluicing the placer or crushed low-grade ore. Insead of developing a superior dry-washing machine (blessed with mechanical simplicity), he decided to use high voltages (immediately introducing massive complexity, and non-maintainability) to separate gold dust from dirt. He patented his invention, it’s here.

Although remembered for his electrical inventions, Edison didn’t really have a clue about the physics of electricity. In 1890 he admitted in court, in front of a Federal Judge, that he didn’t fully understand Ohm’s law when he’d “invented” the light bulb. He certainly didn’t understand the practicalities of using static electricity. In his laboratory he had an X-ray room with a Holtz electrostatic generator -

Above, Edison’s X-ray room, with his Holtz electrostatic generator at left. In order to function, the Holtz machine had to be kept scrupulously free of dust and moisture, which is why it’s enclosed in a glass case. Also in shot is an X-ray tube, a fluoroscope and large Rhumkorff induction coil, and Edison’s assistant Charles Dally, whose brother Clarence had a rather bad time testing Edison’s scheelite light bulbs, but we’ll come to that.
The Electrostatic Separation of Minerals
A Holtz electrostatic generator like Edison’s is capable of producing four inch sparks, that’s well over one hundred thousand volts (100kV). At high voltages like this, materials like wood act like conductors, because of adsorbed water. Also, minerals such as chromite become slightly conductive, enabling a particle of chromite to hold a charge of static electricity. Garnet, on the other-hand, remains stubbornly insulating, and a particle of garnet won’t hold a charge. This enables a mixture of garnet and chromite to be separated -
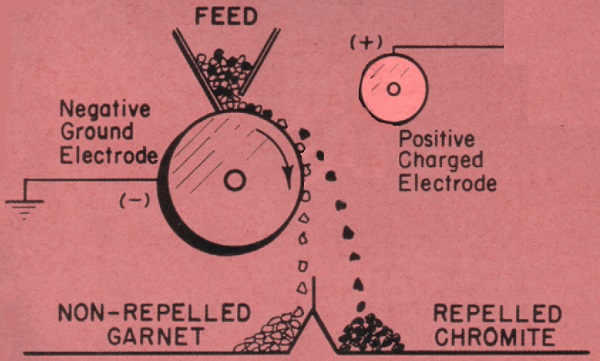
Above, the mixture of chromite and garnet feed onto the surface of a rotating metal drum. The drum is highly negatively charged. An adjacent stationary metal electrode is highly positively charged. When the chromite particles contact the rotating drum, they immediately become negatively charged. Because like charges repel, the particles are repelled away from the drum. Conversely, because unlike charges attract, the chromite is attracted to the positive electrode. The repulsion and attraction alter the trajectory of the chromite as it falls from the roller, and it’s separated from the garnet, which is unaffected by the strong electrostatic field. The highly charged state of the roller and electrode can be accomplished using an electrostatic generator like a Holtz or Wimshurst, an induction coil like a Rhumkorff, or nowadays using an electronic solid-state high voltage unit.
The above only works if the feedstock is completely dry. Even a humid day would cause the garnet to adsorb some moisture, and become conductive. It would then become charged, just like the chromite, and separation would be severely reduced or ineffective. Also, as has been mentioned, the sources of high voltage are themselves highly susceptible to humidity and dust.
As such, it is a demanding process to be exploited commercially, and mineral electrostatic separators are seldom used outside of laboratories. Gold could be very effectively separated using high voltages, as it’s an excellent conductor, but close control of particle size and humidity problems rule out its use. Much better systems exist which simply use the density of gold for separation.
Edison’s Dodgy Hybrid System
Thomas Edison didn’t use the principles outlined above to electrostatically separate his gold. If you read his patent and fathom out what is actually taking place in his diagram of the invention (see a little above), it turns out he doesn’t utilise the high voltage to pluck the gold from the powdered ore, he plucks the waste minerals from the gold.
His invention doesn’t use the difference in conductivity of minerals for separation. What this genius is doing is attempting to levitate the powdered ore with static electricity and, because of the greater density of the gold, separate it in that manner. It’s a shite idea. There are so many variables involved, each of which would inhibit this action. The adsorbed moisture on the belt being the main achilles heel, assuming the feedstock is absolutely dry. Also, in terms of overall use of energy, the electrostatic generators are only a few percent efficient, and the Great Man is expecting them to power the levitation of thousands of tons of powdered waste-rock......
However, he got his contraption working perfectly in his laboratory, through sheer persistence. He was awarded the patent in 1892. In 1897 a sample of ore, from a New Mexico mine he had his eye on, was crushed to a powder and sent through the machine. The gold successfully fell off the end of the belt, and the powdered rock levitated up a little, before falling into the waste catcher.

Buoyed-up by this result in his laboratory, in January 1898 he bought his gold mine. The Ortiz Mine, Santa Fe Co., New Mexico. It had been mined for gold using Indian slave labour by the Spanish around 1680, then forgotten about. Placer deposits were found by sheepherder Jose Francisco Ortiz in 1828, with hard rock being mined five years later. The Ortiz was the first gold lode mine in the West.

When the rich pickings had been extracted, the poor-grade ore that was left, and especially the lack of water to properly wash out the gold, led to a period of dormancy. Enter Edison.
By the summer of 1898 Edison was ready for preliminary testing at the site. Over the previous months, shipments of mill machinery had been delivered by rail and then horse-drawn waggons, and he’d built the mill with its multiple conveyor belt assemblies, bucket elevators, engines, generators, sand screens, and Holtz electrostatic machines. He’d spent $500,000.
It didn’t work.
What appears arid is seldom completely water-free, and the sun-baked ground at the Ortiz unfortunately contained enough moisture to make the levitation machine non-functional. Edison wasn't pleased. The powdered ore would have to be dried before separation.
Once he'd sorted that, the mine was hit by sudden storms, with rain drenching the outdoor mill components and washing sand down the conveyor belts and into the machinery. This caused bearings and gears to fail, with power and conveyor belts snapping. Humidity went up, Holtz machines stopped working.... Then the blizzards hit.
Undaunted, Edison poured another $1.5 million into the mine, mill and equipment, announcing to the world in late 1899, "This process for extracting gold from sand is certainly the biggest thing I ever invented. Near Santa Fe, New Mexico is a region of 10 square miles containing gold worth 800 million dollars!"
It still didn’t work.
Using electrostatics is fine under controlled laboratory conditions. But to expect to go straight from an experimental set-up having an extremely flawed design, to a commercial operation in the wilderness of New Mexico is a tad optimistic. It was never going to work. The concept is wrong, and just keeping a single Holtz machine in operation in a dusty mill is ambitious. Edison closed the mine and mill in 1903.
Thomas Edison's Dip Needle & Agglomerated Magnetite
"Geophysics has progressed from Thomas Edison's dip needle, successfully used at Sudbury, Ontario, early in this century, to satellite-mounted magnetometers." Surface Mining, B.A. Kennedy, 1990.
The above quote from this definitive & weighty tome (1100+ pages), illustrates the perceived connection between Edison and the world’s first geophysical instrument, the dip needle. His use of the dip needle in Ontario, we will address later, but for now it’s his early use of it in New Jersey I’d like to discuss. On perusal of the index of Edison, His Life and Inventions, by Dyer & Martin, 1910 (another definitive work, but definitely a work the Great Man helped sculpt) we find this entry of interest -
Magnetic needle for indicating deposits of magnetic iron ore, Edison’s invention of, 479.
And here’s page 479 from the book, detailing Edison’s use of his invention, the magnetic needle, in his own words, to find huge deposits of magnetite -




It all seems very impressive and, as we shall see, Edison embarked on his greatest endeavour, spending millions, to extract the magnetite. It became known as “Edison’s Folly.”
First though, let’s look at his invention, the magnetic needle. It’s a compass rotated with its axis parallel to the surface of the earth, instead of perpendicular. That’s it. -

Also known as a miner’s compass, dip circle, dip needle, dipping compass, dipping needle, or doodlebug magnetometer, the left hand photo shows a laboratory model from 1900, right hand is an iron-ore prospector using one.
The dip of the earth’s magnetic field was first discovered by Georg Hartmann in 1544. In 1581, after much investigation, compass maker Robert Norman published his pamphlet, “The Newe Attractive” (1720 reprint here), with an illustration of his dip needle -

So, Edison’s “special magnetic needle” was a recognised tool well before Edison invented it. In fact, they’d been used for a couple of hundred years in Sweden, for tracing deposits of magnetite.
Let’s now look at how Edison planned to separate the tiny grains of magnetite from the low-grade ore, using his inventive prowess.
Magnetic Magnetite
As its name alludes, magnetite is magnetic. Strictly speaking it is ferrimagnetic, i.e. it is attracted to a magnet, and it also can be magnetised itself; to become a magnet. When a large body of magnetite is directly under a lightning strike, the huge current of electricity produces an intense magnetic field, which magnetises the magnetite, transforming it into lodestone. It is a rare occurrence, so most magnetite is not magnetised. Edison’s magnetite was sparsely distributed as fine crystalline grains within the host rock, therefore only a minute quantity could ever be magnetised by a lightning strike. But it is magnetic; i.e. it is attracted to a magnet.
Small grains of magnetite occur in almost all igneous and metamorphic rocks. Simply put, the mineral is iron oxide, Fe3O4. However, its ionic formula reveals detail -
Fe2+Fe3+2O4
Chemically it is ferrous-ferric oxide. This complexity leads to its magnetism.
In each unit of its crystal structure, there are two ferric cations Fe3+, one ferrous cation Fe2+ and four oxide anions. They arrange as a cubic inverse spinel group structure, which consists of a cubic close packed array of oxide anions, where all of the Fe2+ cations occupy half of the octahedral sites and the Fe3+ cations are split evenly across the remaining octahedral sites and the tetrahedral sites -

Unit cell of magnetite. The gray spheres are oxygen, green are ferrous iron, blue are ferric iron. Also shown are an iron atom in an octahedral space (light blue) and another in a tetrahedral space (gray). These octahedral and tetrahedral sites are replicated through the structure, but only two are highlighted above.
Magnetite's ferrimagnetic properties arise because the electron spins of the Fe2+ and Fe3+ cations in the octahedral sites are coupled, and the spins of the Fe3+ cations in the tetrahedral sites are also coupled, but in anti-parallel to the former. The net effect is that the magnetic contributions of both sets are not balanced. The resultant magnetic moment leads to ferrimagnetism.
If the above is as clear as mud, well…it’s a complex subject, with a full understanding requiring one to be conversant with quantum dynamics (so that’s me out). Even members of the scientific community have been a little baffled by certain magnetic properties of magnetite for some time, with the “Verwey transition” at last being explained by the work of Edoardo Baldini et al, “Discovery of the soft electronic modes of the trimeron order in magnetite,” published in Nature, 9th March 2020. Phew, it’s too much,... let’s get low brow -
Edison’s Killer Rock-Smasher & BIG MAGNETS
Edison planned to powder his low-grade rock and pull out the magnetite with a magnet. He patented his new invention -
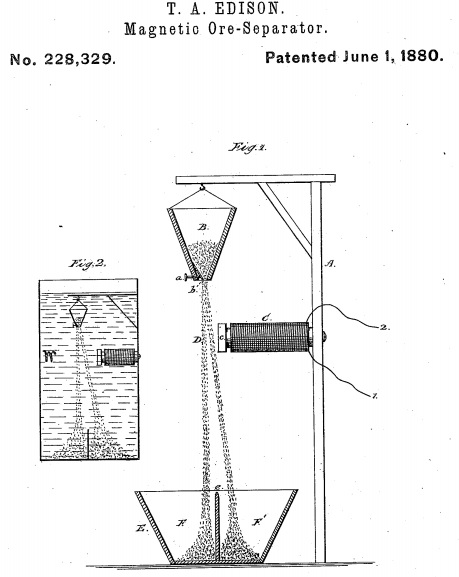
If you’ve made it this far with the article, I hope I’m right in assuming that you immediately grasp what’s going on in the above diagram. If not, the patent is here.
What the hell was the US patent office thinking? Some patentese - “....according to which an invention should be sufficiently inventive - i.e., non-obvious - in order to be patented.” I don’t know about you, but I’d say the above idea is blindingly obvious. Also, since “electro-magnet” is a subset of “magnet,” any prior-art in which a magnet is used, obviously encompasses all forms of magnet. And there’s loads of prior-art; London scrapmen used magnetic separation to remove iron from brass in the 1860s. And as for magnetite -


Bulletin 425, US Bureau of Mines, 1941
Whatever the patent irregularities, Edison poured a fortune into building a monstrous white elephant of a machine, the Ogdensburg Ore Concentrator -

This seven story behemoth, with its three towers rising from the magnetic separator, contained 420 magnets. The initial crushing of the ore was undertaken with a huge pair of contra-rotating (700rpm) toothed iron rollers, the Giant Rolls -

This rock-crusher was designed to handle grand-piano sized six ton boulders. Even today, a mechanical engineer would do the “sharp intake of breath through pursed lips,” given that as a design brief, and having to use the metallurgy of the day. In the following description from Dyer’s Edison biography, it doesn’t sound like it would last long -


Ogdensburg ore processing began in the autumn of 1891. The mill and its machinery unfortunately weren’t up to the task. The huge crushing rollers were not robust enough and often broke loose (at 700rpm!), with catastrophic results. Ore dust penetrated everything, forcing the workers to wear patent masks with pig-like snouts. The bulky and complex steel conveyor system constantly jammed. In the following years, nine workers lost their lives and many more were seriously injured by loose, catapulting machinery. A visiting engineer saw a workman being carried out on a litter, with both legs broken, "Edison looked on grimly and said nothing."
Edison poured $2.9 million (about 60 million quid in today’s real money) into the enterprise, fitting bigger rollers and pins that sheared if a jam occurred, designed to prevent runaway damage to the huge machine, not as an operator safety system. In between breakdowns and fatalities however,... it worked! The magnetic separators produced a pure magnetite sand, ready for the furnaces. The engineering of the magnetic separators is actually very impressive, those interested could download “Electro-Magnetic Ore Separation” by Charles Geofrey Gunther, 1909, from the excellent z-lib.org

Edison’s first shipments of magnetite were a bit of a disappointment at the steel works. The fine magnetite grains just blew up and out of the chimneys. Indefatigable Edison had a fix for this though, he added a binder and formed briquettes (above) from his magnetite sand, adding yet more processes to his system. He referred to the process as agglomeration. The steel works loved them! But not the price.

In 1898 the great Mesabi Iron Range in the northern Minnesota wilderness was discovered. Hundreds of square miles of pure iron ore. It needed only to be open-cast mined and sent directly to the furnaces. Mesabi high-grade ore at $2.75 per ton completely ruined any chance that Edison’s business model could ever succeed. Ogdensburg closed in 1900. Edison commented about his immense financial losses, "it's all gone, but we had a hell of a good time spending it.” The dead men’s families and the mangled survivors probably didn’t think so Thomas…
Edison’s Fluorescent Lamp
This was a really bad invention. Edison had become engrossed with X-rays soon after their discovery by Bequerel in 1895. Bequerel noticed the rays because of the fluorescence of barium platinocyanide Ba[Pt(CN)4]. As well as this synthetic substance, soon it became apparent that X-rays could make various natural minerals fluoresce. The mineral with the greatest brightness on exposure to the new rays was scheelite. Edison had the bright idea of producing the world’s first fluorescent lamp, using X-rays and scheelite. It was lethal.
Scheelite Fluorescence Images for gif courtesy fluomin.com
Images for gif courtesy fluomin.com
Scheelite fluoresces strongly under short-wave ultraviolet light, cathode rays, and X-rays. The reason for the fluorescence was not known in Edison’s time. The fluorescence spectrum is characterised by a broad luminescent band centered at 425 - 435 nm (blue emission) produced by the intrinsic activator, the (WO4)2- anions in the crystal lattice. Some molybdenum is usually present, and the mineral transitions as a solid solution series to powellite CaMoO4. The molybdate anion (MoO4)2- imparts a yellow hue to the fluorescence (but has nothing to do with the frequent yellow colour of most scheelite in daylight).
Molybdenum is an unwanted impurity in tungsten ore, so as a quick check requiring no wet analysis, assay office UV illumination would reveal Mo concentrations -
Blue - pure calcium tungstate
White - 0.35 to 1% molybdenum
Yellow - >1% Mo, doesn't become more yellow after 4.8%
Scheelite's fluorescence spectrum can be further modified by rare earth elements such as terbium replacing calcium cations in the lattice. Such REEs activators need only be in very small concentration to give additional peaks (e.g. 439nm for Tm3+) overlying the base tungstate spectrum.

Scheelite is calcium tungstate Ca(WO4). It crystallises as beautiful yellow octahedra, as in the specimen above, from Edison’s gold mine, the Ortiz, north of the San Pedro Mountains of New Mexico. Unfortunately, while at the Ortiz fiddling with his doomed-not-to-operate gold recovery plant in 1900, Edison was unaware of his scheelite. He was oblivious to the nature of the waste rock, but perhaps if Kunz had included a scheelite specimen as part of his collection....

Instead, he had to buy scheelite for his new lamps. One of Edison’s mates was Buffalo Bill (William Cody), another over-popularised gentleman of the time. Cody owned the gold and tungsten mine, Campo Bonito in the Santa Catalina mountains of Arizona. He’d been conned into buying and developing it by the shady promoter Colonel D. B. Dyer, a former Indian agent, and he was destined to lose a small fortune with it. However, it did have a tabular shaped 15 meters (50 feet) long and 4 meters (14 feet) wide ore body of cerussite, wulfenite and scheelite with waste material consisting primarily of quartz.
With a supply of scheelite in-hand, Edison had his unfortunate assistant Clarence Dally make the world’s first fluorescent lamps. Dally powdered Buffalo Bill’s scheelite crystals, then sintered the calcium tungstate powder into the interior wall of glass tubes. Platinum electrodes were then sealed into both ends of the tubes with a blowtorch. The tubes were then evacuated using a mercury sprengel pump, and sealed.
Dally then attached an induction coil to supply around 20,000 volts, and the lamps lit up,... brilliantly. He began testing the lights, for days on end….. Unfortunately the X-rays affected his hands….
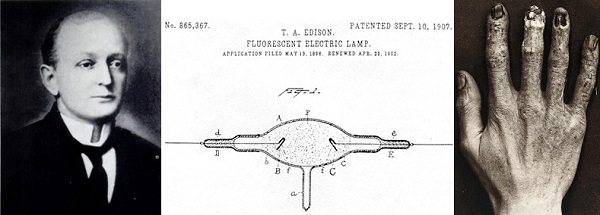
After Clarence Dally had his arms amputated, Edison gave up further work on his fluorescent lamp. After Clarence died, age 39, from mediastinal cancer, Edison had this to say -
"My researches, I might as well tell you now that I have abandoned them, were in the direction of making a fluorescent lamp. I obtained results which brought me each day nearer to the object of my desire. I found a crystal that was fluorescing 12,000 times, and I thought I had my lamp. Then came the question of practical use. I could make the lamp all right, but when I did so I found that it would kill everybody who would use it continuously.”
It wouldn’t be until 1906 when a real scientist & engineer, William Coolidge working at General Electric, developed swaged and sintered tungsten filaments, to replace the fragile carbon filaments invented by Swan. This kickstarted the mining of scheelite for light bulbs that didn’t cause cancer. Weirdly, even though Edison expressed reservations about his fluorescent lamp, he was awarded the patent for this killer application of scheelite in 1907, three years after Clarence Dally died….
Let’s end on another “special magnetic needle” anecdote, with no misery except yet more financial woes for Edison -
“The cobalt battery didn't come up to expectations, Henry.”

Thomas Edison and Henry Ford were buddies. Both massively rich, they embarked on a joint venture to produce electric vehicles. Edison was to provide the batteries. If Edison had been successful on his part of the project, making inexpensive, lightweight and long lasting rechargeable batteries, the world could have been a different place. Greta Thunberg would be smiling and Elon Musk’s Tesla cars wouldn’t be revolutionary. (Aspects of Musk resonate with Edison, he’s a tremendously driven and successful risk taker, but with odd ideas that are irrational non-starters, like hyperloop).
The Edison Storage Battery Company was founded in 1900. Edison went about choosing the right battery chemistry in his plodding, methodical manner, by doing 10,000 experiments with various combinations of substances as anodes and cathodes. Then he chose the invention of Waldemar Jungner, the Swedish inventor and engineer, as his preferred electro-chemistry. Jungner had patented the nickel-iron rechargeable battery in 1899. He’d done this by careful consideration of the electro-chemistry, and a few well thought out experiments, all well before Edison’s scattergun science approach.
Edison’s revolutionary new battery, the nickel-iron rechargeable battery, was developed in the following few years. He tried using lithium hydroxide as an additive to the potassium hydroxide electrolyte. He contacted his minerals man Kunz, who was a spodumene aficionado and knew where folk were mining the mineral for its lithium content, like the Etta mine in South Dakota -


Above, a lithium miner at the Etta mine. His handiwork, the diagonal casts in pegmatite where giant spodumene crystals once lay. Next to him, the cast of a vertically oriented crystal shows a termination much like the small (50cm?) un-mined crystal at upper left.

The lithium helped, but didn’t solve his battery problems. Next he experimented with replacing the iron anode with cobalt. He was convinced this would be the key to solving some of the problems with his battery, such as the high internal resistance. He needed sources of both nickel and cobalt.
Edison decided to do some personal prospecting. In 1901 he and his wife travelled to Falconbridge in Ontario to search for nickel for his batteries. He used the “special magnetic needle,” which indicated a large ore body beneath his feet. Edison staked a 40 acre claim and attempted to sink a shaft. Over the next couple of years the mining was repeatedly halted by a layer of quicksand. He gave it up as a bad job in 1903, and the abandoned claim eventually became Crown land. Edison had missed a rich nickel-copper ore deposit by 15 feet. This didn’t become apparent until 15 years later, after drilling and assay, when Falconbridge Nickel Mines Limited started mining the huge nickel deposits of the Sudbury Basin, making millions from Edison’s old claim..

Edison’s interest in the mineral wealth of Ontario was rekindled however. The same year he gave up his failed nickel mine, two railroad workers stumbled on large silver veins 80 miles away from Falconbridge. By 1905 a full-scale silver rush was underway, and the town of Cobalt, Ontario sprang up to serve as its hub. By 1908 Cobalt produced 9% of the world's silver, and in 1911 produced 31.5 million ounces. The finds were spectacular, for instance the “Silver Sidewalk,” a contiguous mass of native silver up to 0.5m (1.6 feet) wide, 100 metres (328 ft) long and up to 60 (197 ft) metres deep -

But Edison wasn’t interested in silver. He was after the cobalt minerals associated with the silver. The new settlement of Cobalt, Ontario, was named because of this mineralisation. Large plates and leaves of silver ("pieces of native silver as big as stove lids and cannon balls," Miller, 1903) were uncovered from the ground, pink with cobalt bloom, or erythrite Co3(AsO4)2·8H2O. The silver was frequently found as stringers associated with -
- Skutterudite (Co,Ni)As3
- Cobaltite CoAsS
- Safflorite (Co,Ni,Fe)As2
- Glaucodot (Co0.50Fe0.50)AsS
And infrequently found with Cobalt’s type-locality cobalt minerals -
- Langisite CoAs
- Clinosafflorite CoAs2

“Edison sent undercover representatives to Cobalt, Ontario to buy cobalt bearing minerals, which were produced as by-products from silver mining in the area, for his battery manufacturing facility in New Jersey. He also used these representatives to attempt to buy high-grade cobalt silver mines and prospects. Eventually Edison acquired the Darby property (now the Thomas Edison Mine) in 1905. From 1905 to 1907 Edison remotely directed operations at the mine which included sinking two shafts to 150 feet, which were connected by an adit, as well as several exploration drifts and crosscuts. Between 6 and 8 tons of ore of unknown grade are reported to have been extracted, but no commercial production is recorded.” From edisoncobalt.com
The mine doesn’t appear to have done too well, and very little mineralisation is apparent in the dumps. Here’s a look round the Edison mine, courtesy of Greig (meMiner), his dog Daisy and a dodgy camera -
After six years of fruitless experimentation with cobalt, Edison decided to go back to Jungner’s invention, nickel-iron potash batteries. In 1909 he remarked that it had taken him "seven years and $1,750,000 to find out how to make a battery that will last four years." Ford didn’t pull the plug on the electric vehicle project until 1914. Nickel-iron batteries just weren’t suitable in terms of discharge characteristics, energy storage/weight, or cost, for the Ford-Edison EV, and the project was shelved. However nickel-iron batteries are robust and long-lived, and found many decades of use down mines worldwide -

Edison’s cobalt mineral interests in Ontario are coming to fruition however…
Elon Musk is desperate for cobalt. His Tesla cars use lithium-ion battery technology, these batteries need cobalt as part of the cathode electro-chemistry (LiCoO2) to have high discharge performance. Elon gets his cobalt from the Congo -

Elon’s battery production gigafactories need a source of supply of cobalt that’s not tainted with human misery. An estimated 150,000 artisanal cobalt miners work in pretty wretched conditions in the Democratic Republic of Congo. Whilst seeking to use cobalt-free batteries in the future, Tesla has committed to sourcing its cobalt entirely from North America.
Edison Cobalt Corp is a Canada-based junior mining exploration company. It acquired the property of the old Edison mine, together with adjacent mines. Cores drilled in the last few years have shown cobalt values up to 3.66%. Thomas Edison was on the right track… but missed again.

We’ll end with a beautiful scheelite crystal from Thomas Edison’s gold mine, as elusive to the Great Man as Falconbridge's nickeline, Cobalt's skutterudite and Ogdensburg health and safety concerns -

References -
George Frederick Kunz, Wikipedia
George English, Mineralogical Record Biographical Archive
The Scientific Valuation of Minerals, George Letchworth English, 1927, American Mineralogist
Edison Laboratory Furnishings Report. US Dept. of the Interior, 1995
US Patent 476991 Method for Separating Ores, 1892
Surface Mining, B.A. Kennedy, 1990
Edison, His Life and Inventions, by Dyer & Martin, 1910
The Newe Attractive, Robert Norman, 1720
Discovery of the soft electronic modes of the trimeron order in magnetite, Baldini et al., Nature, 9th March 2020
US Patent 228329 Magnetic Ore Separator, 1880
Bulletin 425, US Bureau of Mines, 1941
Electro-Magnetic Ore Separation, (z-lib.org), Charles Geofrey Gunther, 1909
And Now For Something Completely Different