
Following on from reading about pit-men discovering very substantial (6m long, 3m high and 1m wide) vugs in the witherite at the Morrison Busty Colliery, I’ve uncovered another spectacular occurance of walk-in “crystal caves in coal mines.”
About 56 million years ago, huge volcanic activity was occurring in the Scottish Hebrides. Upwards of 850 km3 of molten rock rove the earth apart, in a series of vertical magma filled cracks, or dykes. These ribbons of flowing red hot magma travelled across country to the North East England coalfield. When a vertical dyke passed through the horizontal beds of the coal measures, the coal was roasted to coke, for several meters either side of the dyke. Having lain undisturbed for hundreds of millions of years, the coal was subjected to intense heat from the intrusion of magma, flowing from 400 km away. Because this took place far underground, there was obviously no oxygen, so the destructive distillation of coal took place.
The destructive distillation of coal was a big thing when I was a child, living in an area dominated by coal mining. The gasworks were huge, very smelly operations, where coal was railed in, put into airtight iron retorts, and heated to red-hot by a furnace, itself fuelled by one of its own process products - coke. Gas and vapour were evolved in abundance. The coal gas was scrubbed with water to remove tars and ammoniacal gases, then passed over bog iron ore. The purified coal gas has this approximate composition -
- hydrogen 50%
- methane 35%
- carbon monoxide 10%
- ethylene 5%
Bog iron is one of the most unappealing mineral assemblages. Seen in few collections, its bland, rusty and amorphous appearance offers zero aesthetic value.

A couple of lethal constituents of coal gas, hydrogen sulphide and hydrogen cyanide, were removed from the gas using bog iron. The iron oxyhydroxides constituting the bog iron, commonly goethite (FeO(OH)), react with these poisonous gases, producing iron sulphide and a rather unusual chemical mix, called by gas workers “Blue Billy.” This stuff was a difficult pollutant, especially when the bog iron was used with lime in the purifier (below).

Blue Billy is primarily composed of ferric ferrocyanide, or Prussian Blue, Fe4[Fe(CN)6]3. This compound was used as an artist’s pigment, and superseded the extremely expensive ultramarine, made from lapis lazuli (an Afghani specimen of which is below).

A completely non-mineralogical excursion here - but I had to add this, for anyone who's read my previous post "Terra Ponderosa, Annfield Plain Witherite & Fat Man." -
The production and use of coal gas was developed by William Murdoch in 1794. His engineering recognition, career, and wealth, although not inconsiderable, were blunted by the advice and actions of one man. This man recommended that Murdoch shouldn't bother patenting his invention of producing gas from coal for illumination. Also, to increase the stature of his father, Murdoch's boss, this man destroyed communications from Murdoch which detailed his inventions in steam technology, patents for which were taken out by the boss. These despicable underhand dealings were the work of James "Dog-Killer" Watt.
Apologies for this diversion, back to dyke baked coke -
84lbs (one cubic foot) of coal from the Felling Main seam (for an example), produces 420 cubic feet of gas when pyrolysed. So when the dykes hit coal seams, every cubic foot of coal that was reduced to coke, generated 420 times the volume of gas. Instead of diffusing back into the coal seam, this gas would occasionally be injected into the magma to form a gas-filled void. Also, large sections of the coal seam could break off, and become engulfed by the magma, there to be coked and similarly, cause a large gas bubble. These giant vesicles are of a completely different nature to the amethyst lined geodes of Rio Grande do Sul, Brazil.
The South American giant cavities in basalt are formed by outgassing. That is, when the magma gets near the surface, pressure is reduced, and dissolved gases such as water vapour and carbon dioxide are released. This forms vesicular basalt. The giant vesicles are vertical in nature; 3m wide by 6m tall. Much later mineralisation by silica and manganese rich hydrothermal fluids produced the “amethyst cathedrals,” specimens of which are seen in most rock shops.

The Northern England giant cavities in basalt are not formed by outgassing from the magma. After travelling 400 km from the eruptive site in the Hebrides, there aren’t any dissolved gases left in it. They’re formed by coal gas. The giant vesicles are linear in nature, going with the flow; 22m long by 3.7m high and 3m wide. Much later mineralisation by hydrothermal fluids produced a dogtooth calcite, marcasite and pyrite crystal lining. Specimens formed in coal gas vesicles are never seen in rock shops.

In 1952 coal miners down West Sleekburn Colliery, Bedlington, Northumberland, drove a passage through a tholeiite basalt dyke, which intruded the coal seam they were working, they broke into a huge crystal filled cavity.
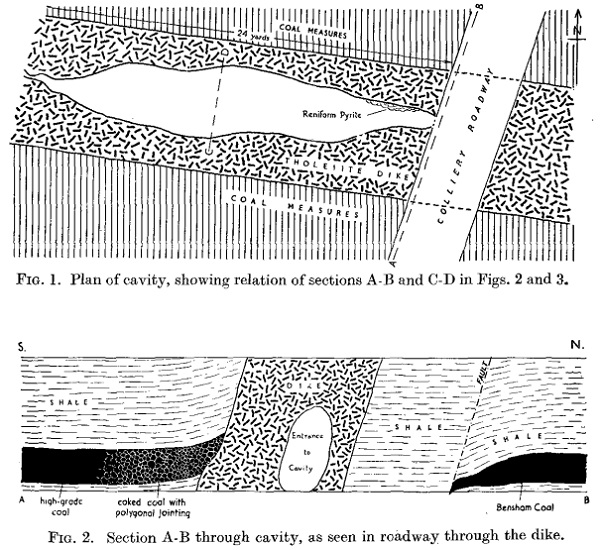
The manager and under-manager at the colliery were the first to enter the cavity. They nearly lost consciousness, it being filled with what they later described as marsh-gas, or methane. Specimens from the cavity were examined by Bert Randall F.R.S. who described the mineralisation excellently in a paper published in Mineralogical Magazine 1953, illustrations from which are shown above.
The cavity wasn’t unique -

Randall describes the mineralisation comprehensively, here's a couple of excerpts -
“The minerals developed in the cavity are : pyrite, marcasite, ankeritic
calcite, and a magnesium-rich ankerite…… Carbonate stalactites are developed mainly in the eastern third of the cavity, the largest being about two feet in length. In the last accessible portion of the cavity are some remarkable stalactites of carbonate coated with botryoidal pyrite…… The radial structure of the growth is obvious in a hand-specimen, and most of the surface shows an iridescence with blue and green tints.”
Since Thatcher closed the pits in Britain, it’s unlikely that any more coal gas giant vesicles will be found. Unless, elsewhere in the world, there are other examples of working coal measures which have been intruded by dyke swarms.
As an end note, you may have wondered why bog iron was mentioned in a little detail, and what was that about Blue Billy? A little speculation, no more…. The coal gas forming the giant vesicles in tholeiitic basalt dykes hadn’t been scrubbed. Rich in hydrocarbons such as tars, and containing appreciable quantities (0.1%) of hydrogen cyanide, these “contaminants” had to go somewhere, over the millions of years since the chamber was formed. If mineral samples taken from the West Sleekburn colliery in 1952 were re-examined, with today’s analytical tools at hand, the highly unusual environment could be found to have formed some exotic minerals……
The Carbon Mineral Challenge could be very interested in any organic compounds formed from the witches brew of hydrocarbons in the virgin (i.e. unpurified) coal gas. There’s also hydrogen sulphide, hydrogen cyanide, cyanogen, ammonia, and with water vapour, ammonium, thiocyanate and ferrocyanide salts. Mix with iron sulphide from entrained coal, leave for several tens of millions of years….. When Bert Randall F.G.S described the crystallisation in the West Sleekburn cavity, he would be well aware of the appearance of colourful iridescent films on iron minerals. Yet he thought the iridescent films on pyrite and marcasite unusual enough for (inconclusive) testing, “The iridescent surface of the sulphide material suggested the presence of copper or nickel, but this was not substantiated by qualitative analysis.” Blue Billy?
References -
A large mineralized cavity in a tholeiite dike in Northumberland, Dr. Bert Randall, 1953
A treatise on gas-works, Samuel Hughes, 1853
The History and Operation of Gasworks, pdf download, Dr Russell Thomas, 2014
The Carbon Mineral Challenge
Bert Randall BSc., F.R.S., Obituary 2012
And Now for Something Completely Different
_______________